MedicalBazzar
Biofeedback Nerve and Muscle Stimulator KM531
Biofeedback Nerve and Muscle Stimulator KM531
Couldn't load pickup availability
Specification & Details
Specification & Details
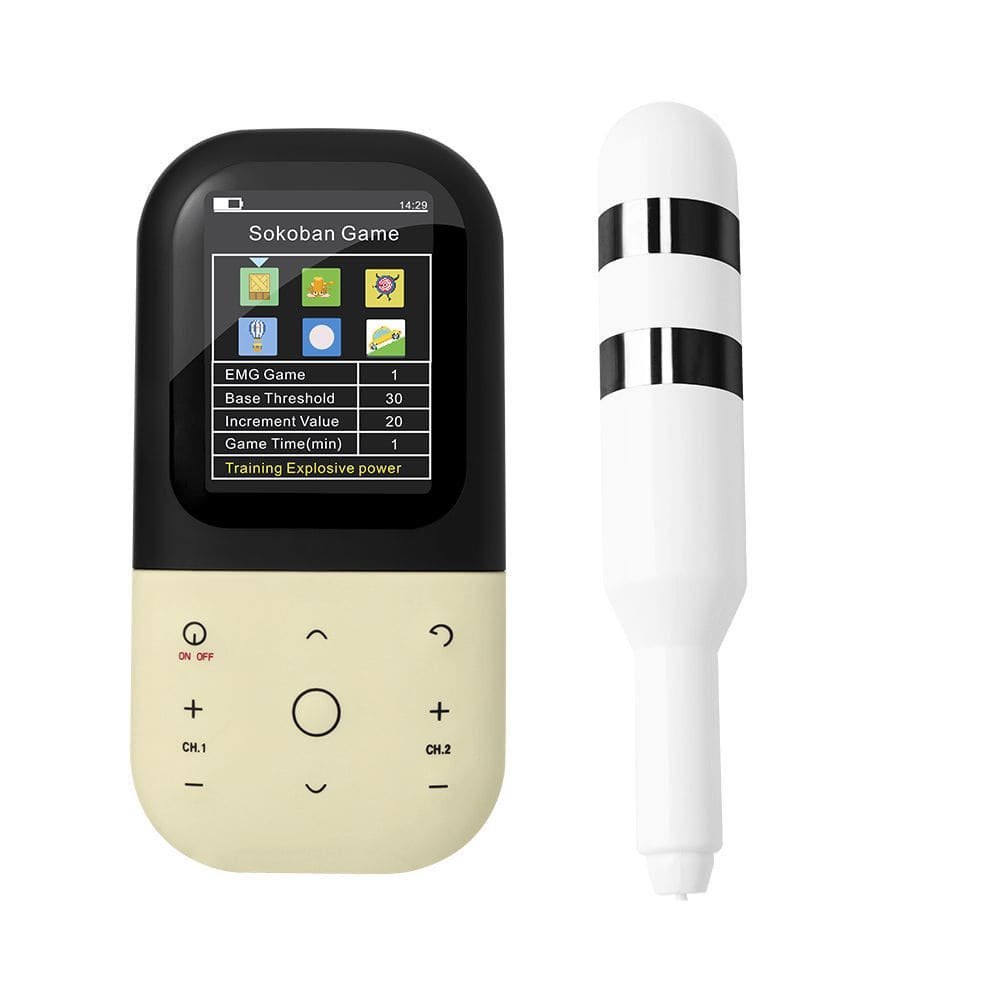
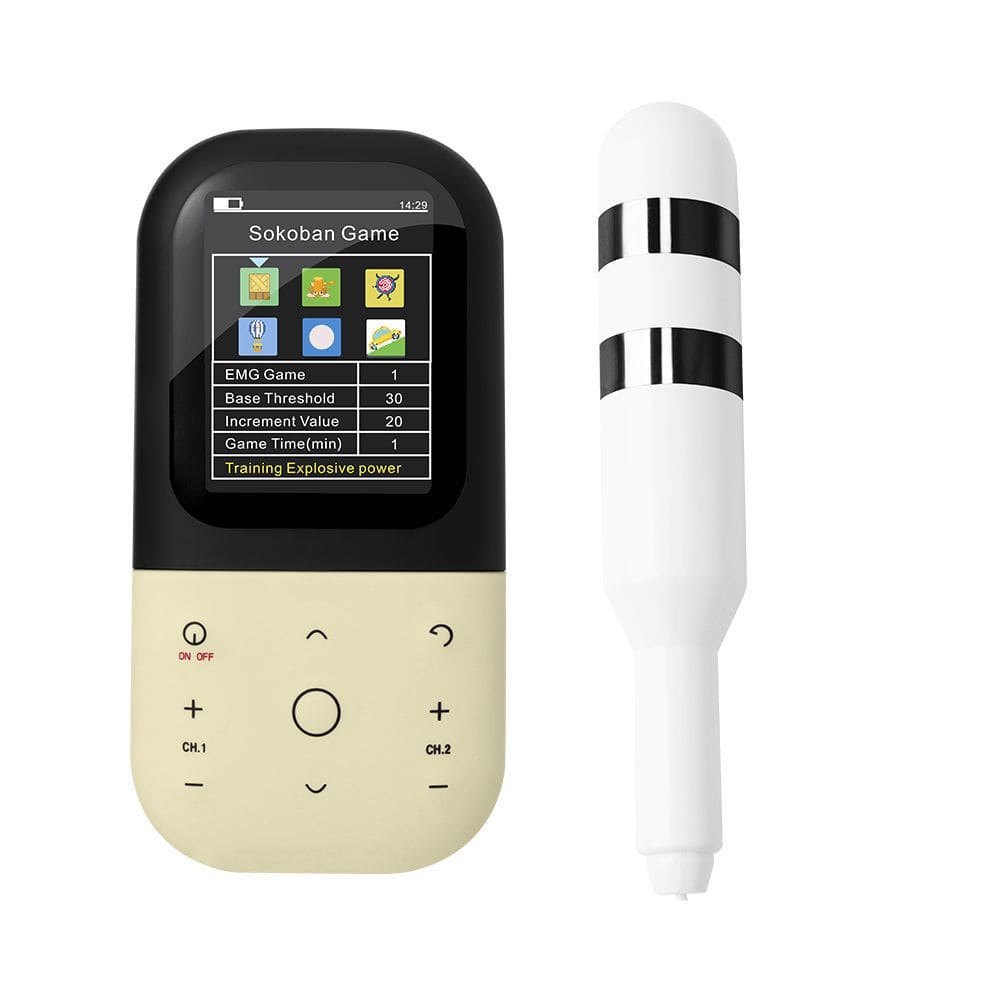
KM531 EMG biofeedback stimulator with vaginal and anal probe for muscle training - Physical therapy pelvic floor muscle Kegel exerciser/ rehabilitation biofeedback devices
1. Introduction
This Biofeedback device is a new type of biofeedback and neuromuscular electrical stimulation therapy device for patients with muscle dysfunction through the evaluation of myoelectric signal acquisition, multimedia biofeedback training, electromyography triggered electrical stimulation, passive electrical stimulation training and treatment. Perform routine muscle training, combined with individualized electrical stimulation therapy, awaken and activate muscles, accelerate the recovery of muscle tone and elastic, and have a good effect on preventing and treating muscle disorders. Features are as shown below:
Four operation modes (EMG Test, EMG Game, ETS, and STIM) have been set up to assist patients in exercising.
EMG: Electromyography
ETS: Electromyography triggered stimulation
STIM: Neuromuscular stimulation
Independent dual-channel EMG signals acquisition. EMG data of multiple sites are obtained simultaneously to provide basis for treatment.
Independent dual-channel electrical stimulation output, convenient for the treatment of different sites or complete the treatment in coordination.
Ergonomic design, effectively prevent the vaginal/anal probe off and rotation, to ensure that treatment effect.
All patient-contacting materials are tested and passed the bio-compatibility according to ISO10993-1 requirements.
2. Indications for Use:
The device is for home use and the intended operator is patient who has muscle dysfunction.
For EMG:
To determine the activation timing of muscles for:
1) Retaining of muscle activation
2) Coordination of muscle activation
An indication of the force produced by muscle for control and maintenance of muscle contractions.
3) Relaxation muscle training
4) Muscle re-education
5) EMG Game for training muscle power, explosive power and speed.
For EMG triggered Stim:
1) Stroke rehab by muscle re-education
2) Relaxation of muscle spasms
3) Prevention or retardation of disuse atrophy
4) Increase local blood circulation
5) Muscle re-education
6) Maintaining or increasing range of motion
As nonimplanted electrical continence device:
The device is a non-implanted muscle stimulator designed to provide electrical stimulation and neuromuscular re-education for the purpose of rehabilitation of weak muscles, pelvic floor muscles and restoration of neuromuscular control during the treatment of urinary continence.
3. Adverse Reactions
These kinds of stimulations have been used for many years to stimulate muscle and nerve fibers to treat a number of muscle and nerve related conditions. Over the last 30 years numerous clinical trials and papers have been written. Patients should stop using the device and should consult with their physicians if they experience adverse reactions from the device.
4. Conformity Standards
IEC 60601-1-2 Medical Electrical Equipment --Part 1: General Requirements for Basic Safety and Essential Performance
IEC 60601-1: Medical Electrical Equipment --Part 1-2: General Requirements for Basic Safety and Essential Performance --Collateral Standard: Electromagnetic Disturbances --Requirements And Tests
IEC 60601-1-11Medical Electrical Equipment --Part 1: General Requirements for Basic Safety and Essential Performance --Collateral Standard: Requirements for Medical Electrical Equipment and Medical Electrical Systems Used in the Home Healthcare Environment
IEC 60601-2-10Medical Electrical Equipment --Part 2-10: Particular Requirements for The Basic Safety And Essential Performance Of Nerve And Muscle Stimulators
IEC 60601-2-40Medical Electrical Equipment --Part 2-40: Particular Requirements for the Basic Safety and Essential Performance of Electromyographs and Evoked Response Equipment
ISO 10993-5Biological Evaluation of Medical Devices –Part 5: Test for In Vitro Cytotoxicity
ISO 10993-10 Biological Evaluation of Medical Devices –Part 10: Test for Irritation and Skin Sensitization
5. Package Content
1 * Main unit
3 * Electrode patches
1* Vaginal probe
1 * Anal probe (optional)
2 * Electrode wires (white)
1* REF signal wire (black)
1* USB wire
1* User Manual
OFFERS
OFFERS
Get 8%Off on all Prepaid Order above Rs 1000/-, Max Discount Rs 800
Use Code - MB8PREPAID
Share